Quality
The Production of Bioactive Collagen Peptides
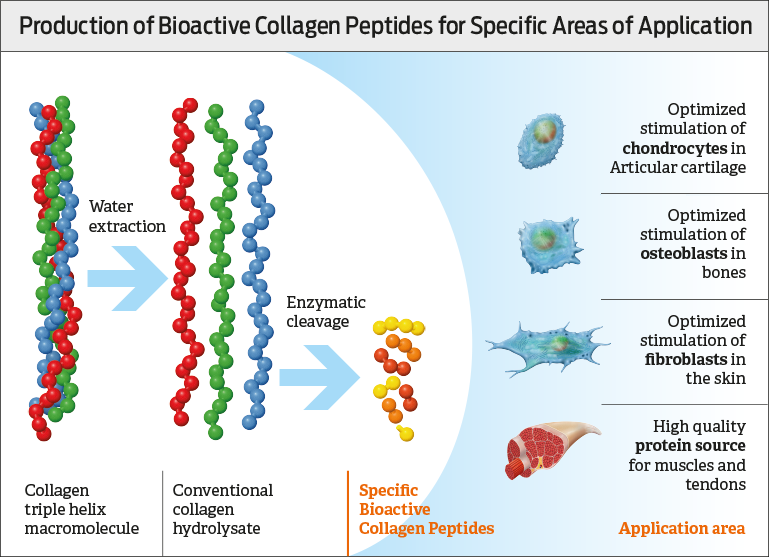
Collagen is an animal protein, and can therefore only be obtained from animals. Collagen peptides are produced from collagen-rich tissues, such as the skin, connective tissue, and bones of pigs, cattle or poultry, as well as from skin, bones, and fins of fish. The properties of the end product are largely determined by the animal species, the age of the animal and the type of collagen predominant in the source tissue [Gòmez-Guillén et al. 2011]. At the beginning of the production, the starting material is washed, homogenized, and demineralized. Water extraction is used to generate gelatine thereof, which is then treated by enzymatic hydrolysis that produces specific peptides according to the enzymes and hydrolysis conditions applied [Daliri et al. 2017, Gòmez-Guillén et al. 2011, Dybka et al. 2009].
The desired peptide fraction is isolated and dried by additional purification and concentration procedures, which lead to products with defined properties. The production conditions significantly affect the efficacy and tolerability of the final product [Daliri et al. 2017]. The efficacy of so-called Bioactive Collagen Peptides (see figure) was tested through clinical studies.
How to Compare Collagen Hydrolysate Products?
Does the Molecular Weight Predict the Effect?
Collagen hydrolysates can be produced from various animal species (pig, cattle, fish) and organs (skin, bones, connective tissue), which results in large variations in the composition of the starting material. In addition, production procedures seem to have an important impact on the effect of the final product. In a clinical study, Kumar and colleagues compared the effect of collagen hydrolysates from pig skin with those from bovine bones. Both preparations were produced with the same method, and no difference was found concerning their effect [Kumar et al. 2015]. On the other hand, Shadow et al. investigated the peptide composition of various hydrolysates derived from either fish or pig. In terms of number and types of peptides, the authors described significant differences between hydrolysates from fish and pig, as well as between two preparations derived from fish. This leads to the conclusion that the effects of one particular collagen hydrolysate product cannot be automatically presumed for another one [Schadow et al. 2017].
The influence of different production conditions on the active properties of several chicken collagen hydrolysates was investigated by Offengenden and colleagues [Offengenden et al 2018]. The authors could show that some properties, such as antioxidant or anti-inflammatory effects, may occur individually or together, depending on the manufacturing conditions. Other properties, such as the stimulation of fibroblasts or the new synthesis of collagen, are peptide-dependent rather than universal properties.
Molecular weight: While gelatine has an average molecular size of about 100 kDa, enzymatic hydrolysis produces peptides with a molecular weight of 0.3 to 8 kDa [Sibilla et al. 2015]. Since the average weight of an amino acid is about 110 Da, collagen hydrolysates contain a mixture of peptides with 2 to 80 amino acids. The average molecular weight is a key characteristic of each preparation. It describes the average molecular size of all present peptides, but does not specify them. As the biological characteristics of hydrolysates are mainly due to their specific peptide composition, the average molecular weight does not serve as a measure for their bioavailability or possible effects.
Collagen hydrolysates are multi-compound mixtures of hundreds of different peptides, which can influence each other in the effect. Clinical studies are therefore needed for a sound comparison of different hydrolysates.
Uptake of Bioactive Collagen Peptides
Bioactive Collagen Peptides usually consist of 3 to 20 amino acids that are linked by a peptide bond. To be ingested without alteration, collagen peptides must be resistant to the acid and protein-degrading enzymes within the gastrointestinal tract. In some cases, Bioactive Collagen Peptides are embedded within a larger, high molecular peptide, and are only released in the gastrointestinal tract [Lemes et al. 2016]. Within the intestine, small peptides of two or three amino acids (di- and tripeptides) are transported from the enterocytes to the bloodstream by a peptide transporter (PEPT1) [Hettiarachchy (Ed.) (2012)].
Not only small peptides, but also larger peptides may be absorbed without prior degradation, either by direct penetration of the cell membrane, by endocytosis, or by diffusion between cells via tight junctions [Hettiarachchy (Ed.) (2012)]. A study in rats showed that collagen hydrolysates derived from pig skin with an average molecular mass of 5.000 Da were predominantly absorbed as di- and tripeptides. Besides those, a significant amount of high molecular peptides entered the systemic blood circulation as well. This means that larger peptides can resist enzymatic cleavage and be absorbed via the intestine [Osawa et al 2018]. Since the helical structure and compact form of these larger peptides is retained, paracellular transport via tight junctions could be considered as a possible mechanism [Wang et al 2019].
Bioactive Collagen Peptides have a unique structure and composition. Their peptide chains are characterized by repeated amino acid sequences composed of proline-hydroxyproline-glycine, which leads to an increased stability against peptide-cleaving proteases. As a result of this, peptide fragments are preserved and may be absorbed intact into the body inducing biological effects on their target cells. They might serve as messenger molecules and stimulate the body’s own repair mechanisms in the event of damage to the extracellular matrix [Liu et al 2015]. This suggests that different production methods might lead to the production of hydrolysates with certain biological functions [Chakrabarti et al 2018]. Admittedly, those effects still have to be confirmed in clinical studies.
Last update: August 2022