Ligaments and Tendons
Structure and Function

Ligaments and tendons are composed of connective tissue including collagen fibers. While ligaments attach bone to bone and thus help stabilize the joint (e.g., the cruciate ligaments of the knee), tendons connect muscles with bones to transmit muscle force and enable movement of the body (e.g., Achilles tendon) [Woo, Levine 1998]. Further examples include the periodontal ligaments, which attach the teeth to the jawbone, or the tendons that connect the eye muscles with the different parts of the eye.
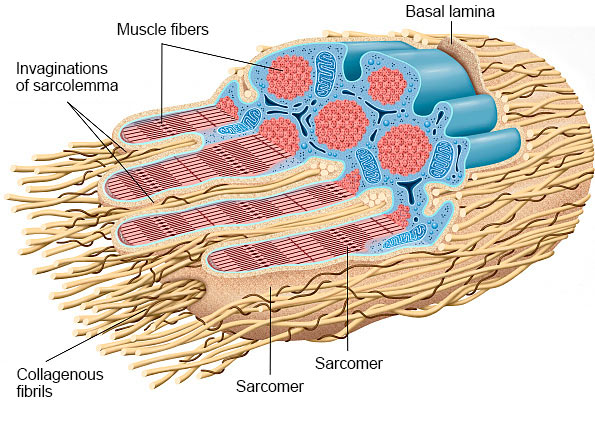
At the end of all skeletal muscle fibers, the collagenous fibrils of the tendons penetrate into the deep finger-shaped indentations of the sarcolemma (cell membrane of a muscle fiber). Because of this, they attach to the basal lamina of muscle fibers over a large area. The tendons are fixed to skeletal muscle fibers by adhesion molecules such as fibronectin. The Golgi tendon organs are located at the junction of tendon and muscle fiber and transmit information about the tension in the muscle to the central nervous system.
Ligaments and tendons have a similar structure, with bundled collagen fibers combined into larger units. However, ligaments and tendons differ in the size of their collagen fibrils and in their organization. They both contain extracellular matrix (ECM) components, water, and fibroblast-like cells called tenocytes which synthesize collagen and further ECM components.
This structure determines the mechanical properties of ligaments and tendons. It allows for adaptation to different loads [Zabrzynski et al. 2018], absorbs shocks, and uses sensory receptors to prevent overloading of the muscles and tendons. However, the energy metabolism of ligaments and tendons is quite slow and the adaptation to movements and loads is delayed compared to that of muscles and bones [Zabrzynski et al. 2018].
Damage to Ligaments and Tendons
Injuries in ligaments and tendons are very common and can occur from rupture, overuse, or inflammatory and degenerative alterations [Wu et al. 2017]. Damage results in swelling, bruising, and loss of strength. The specific cause quite often remains unclear, as several factors usually contribute and degenerative changes often start long time before their symptoms occur [Oliva et al. 2016]. Possible causes include the genetic disposition, age, body weight and diet, injuries, repeated overloading in certain sports, and hormone changes due to metabolic diseases such as diabetes or functional disorders of the thyroid gland. In addition, chronic lack of exercise or the intake of certain drugs can also contribute to their degeneration [Oliva et al. 2016, Zabrzynski et al. 2018]. The degeneration process is considered as a failure in the adaptation and remodeling of the tissue due to the imbalance of degradation and regeneration of collagen and other components of the extracellular matrix [Wu et al. 2017]. As a result, the structure of collagen is less organized and its biomechanical properties change. Furthermore, deposits of fibrin and fat, as well as a calcification process contribute to the progression of degeneration [Wu et al. 2017].
Healing Process
Ligaments and tendons are able to heal, at least in theory. The general process is similar to that of wound healing and is controlled by tenocytes and the surrounding ECM. The initial inflammatory phase is followed by a proliferation phase with synthesis of collagen III. The remodeling phase starts six to eight weeks after the injury and can take up to two years. During this phase, the initially formed collagen III is converted into collagen I, which is cross linked and more resilient to stress. Notably, the healed structure usually does not reach the quality of an unharmed tendon [Wu et al. 2017]. After the inflammatory phase, it is important to expose the tendon to dosed loads, which stimulates collagen formation and avoids tendon atrophy. The load capacity must not be overstrained and sufficient recovery time is crucial to a successful regeneration. Mechanical stress is especially important in order to prevent fixed adhesion of the tendon to its sheath [Zabrzynski et al. 2018].
Effects of Bioactive Collagen Peptides on Tendons and Ligaments
Several experiments have shown that Bioactive Collagen Peptides can influence the biosynthesis of collagen and other ECM components [Minaguchi et al. 2005, Schunk and Oesser 2013, Shaw et al. 2017]. Collagen peptides were shown to increase the synthesis of collagen I and III, as well as elastin, to foster the fibril size, and to change the composition of ECM components. These results suggest that oral administration of Bioactive Collagen Peptides can improve the biomechanical properties of tendons and ligaments.
Clinical Studies
In a case series, Gonçalves reports an improvement of joint pain, resulting from osteochondral lesions, in three athletes by taking 10 g Bioactive Collagen Peptides daily. The treatment of the athletes had covered multimodal therapy with physical rehabilitation and the healing success was confirmed with imaging techniques [Gonçalves 2017].
In another prospective, randomized double-blind study, 160 young women and men suffering from load-induced joint pain received either a daily dose of 5 g Bioactive Collagen Peptides or a placebo. A statistically significant decrease in pain intensity, compared to that reported by the placebo group, was found after 12 weeks [Zdzieblik et al. 2017].
Dressler an co-workers conducted a randomized, double-blind, placebo-controlled study to analyze the influence of Bioactive Collagen Peptides in athletes with chronic ankle joint instability. Notably, ankle injuries are among the most common injuries and result in most of the cases from strain or rupture of one or more ligaments. These injuries can lead to chronic instability of the joint, which increases the risk of further injuries and impairments not only in athletes. The degree of impairment was assessed by standardized procedures (Cumberland Ankle Instability Score CAIT, German Version of the Foot and Ankle Ability Measure FAAM-G), and the mechanical stability was analyzed by arthrometry. Each participant of the study received 5 g Bioactive Collagen Peptides or a placebo daily for 6 months. The collagen peptides were taken within one hour after stress training, which consisted of 5 minutes jumping rope, 15 knee bends, and 15 one-legged heel raises. The exercise program was scheduled for every other day, with one day rest in between. The test product (Bioactive Collagen Peptides) was to always be taken at the same time, regardless if it was exercise day or rest. The participant’s diet and further physical activity were kept as usual, and all participants were interviewed three months after the end of the study. After six months, the assessment by CAIT and FAAM-G showed statistically significant improvements in participants with Bioactive Collagen Peptides compared to the placebo group. The mechanical stability of the ankle measured by arthrometry was only slightly improved in the verum group, but deteriorated in the placebo group. Furthermore, three months after completion of the study, the frequency of injuries was decreased in a statistically significant manner in the verum group [Dressler et al. 2018].
Praet et al. investigated the influence of Bioactive Collagen Peptides on the symptoms of Achilles tendinopathy in a small but very well designed pilot study [Praet et al. 2019]. This study included 20 patients (13 men, 7 women) with long-standing Achilles tendinopathy in the middle region of their Achilles tendon on one or both sides. Ten participants received 2.5 g Bioactive Collagen Peptides or placebo (maltodextrin) twice daily. The treatment was reversed after three months, i.e., the group that had first received verum was then given placebo and vice versa (crossover study design). This design offers the advantage of a minimized effect of interindividual differences on the result. In addition, the patients were told to complete a well-structured training program. Several parameters were investigated: changes in pain and function of the Achilles tendon (standardized questionnaire, VISA-A score), patient satisfaction, the possibility to resume running training, changes in the microvascularity of the tendon, and safety. Although the study was too small to reliably detect differences between placebo and verum, an improvement in clinically significant parameters (VISA-A score, resumption of running training) was detected during treatment with Bioactive Collagen Peptides. Microvascularity decreased similarly in both groups, regardless of placebo and verum. The authors concluded that the intake of Bioactive Collagen Peptides can support the benefits of well-structured training.
In summary, the intake of Bioactive Collagen Peptides has a positive effect on the stability and resilience of joints. However, dosed stress stimuli and adequate rest periods are crucial for joint regeneration. As adaptation of tendons and ligaments to stress is delayed, an improvement can only be expected in the long term. Of particular clinical significance is the reduced frequency of new injuries after a 6-months use of Bioactive Collagen Peptides and the reduced symptoms of tendinopathies. The optimal time of administration is still unclear as different studies used different dosing regimens. In the study of Praet et al., peptides were taken 30 minutes before exercise whereas peptides were taken up to 60 minutes after exercise in the study of Dressler et al. Altogether, the results still have to be confirmed in larger, controlled studies.
Source of figure: Frank Geisler (MediDesign)
Last update: January 2023