Muscles
Structure of Skeletal Muscles
Skeletal muscles or striated muscles are connected to the skeleton by tendons and allow for movement of the body. The activity of striated muscles is voluntarily controlled, with the exception of the heart muscle, which is striated but involuntarily controlled.
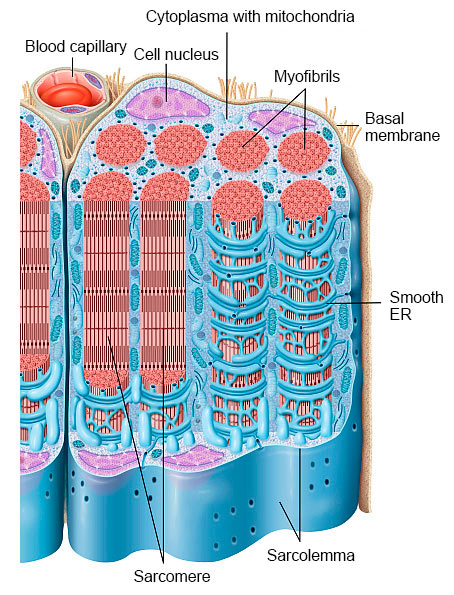
A muscle fiber is a polynucleated cell that can be more than 15 cm in length. Each muscle fiber contains several myofibrils bundled together in a lengthwise direction. Each myofibril consists of sarcomeres which represent the smallest functional structure of muscle fibers. Myofibrils are enveloped by endoplasmic reticulum (ER) which holds the calcium ions that are required for the contraction of the muscle fiber. The actin and myosin filaments in the sarcomeres perform the contraction of muscle fibers.
Each muscle fiber is surrounded by a layer of connective tissue called endomysium. Several muscle fibers are combined into bundles, which are then surrounded as a whole by connective tissue (perimysium). Many of these encased bundles come together to form a muscle which is surrounded by a very strong connective tissue called facia (epimysium). At both ends of the muscle, the fascia continues with the tendon.
Plasticity of the Muscle
Skeletal muscles can significantly adapt to training requirements and stress [Steinacker et al. 2002]. This adaptation process to various external stimuli is referred to as plasticity of the muscle [Steinacker et al. 2002] and is mainly controlled by protein metabolism within the muscle itself. Protein metabolism is influenced by the protein content of the ingested food, and by the body’s transport mechanisms for amino acids [Dickinson et al. 2013]. In addition, the connective tissue surrounding the muscle is subject to adaptation and influences protein synthesis via mechanical stimuli. There is presumably a close correlation between muscle and connective tissue [Narici et al. 2007].
Lack of exercise, aging, and chronic inflammation lead to a decrease in both muscle mass and strength. The energetic situation and in particular the levels of ATP and creatine phosphate are important signals and regulators of metabolism. Nutrition and recovery are, therefore, crucial during training and adaptation [Steinacker et al. 2002].
The biological fitness of muscles is also important for body health. Many studies have shown a decreased risk for chronic diseases, e.g., cardiovascular diseases, stroke, high blood pressure, metabolic syndrome, or type 2 diabetes mellitus, in individuals with increased muscle strength [Strasser et al. 2018].
Effects of Age and Lack of Exercise
From the age of 40, muscle mass and strength decrease by about 1% per year. However, interindividual differences vary considerably depending on the individual aging process and amount of physical activity. These parameters influence muscle architecture (e.g., decreased number of muscle fibers and muscle size, changed ratio of fiber types), mechanical properties of surrounding connective tissue, and blood supply. Immobilization and lack of activity change muscle size as well as the surrounding connective tissue, whereas the number of muscle fibers remains constant. However, the number of muscle fibers decreases as a result of the aging process [Strasser et al. 2018]. The combination of advanced age and sedentary lifestyle leads to increased muscle loss and often ends in a vicious cycle. The loss of muscle mass and strength due to aging is called sarcopenia [Robinson et al. 2017].
Effects of Physical Activity
Strength training increases both muscle mass and strength; endurance training strengthens cardiovascular fitness. Strength training leads to increased protein synthesis within the muscle. Muscle precursor cells (so called satellite cells) are developed and activated to form new muscle fibers. Endurance training, in contrast, leads to increased blood supply and energy utilization, especially of fat [Strasser et al. 2018]. The combination of both types of training provides the best results in terms of health and fitness.
Effects of Food
As the synthesis of muscle cells requires amino acids or protein, their supply can be a limiting factor. Therefore, a higher protein intake is recommended especially for people of advancing age, as they generally metabolize nutrients less well than younger people [Strasser et al. 2018, Robinson et al. 2017]. Protein intake after the training can support the formation of muscle mass, provided that the exercise is appropriate. The intake of 20 to 25 g protein after strength training is recommended to promote muscle regeneration and protein synthesis. For older people, even 40 g protein is recommended. As these amounts are quite high, a protein-rich meal 90 to 120 minutes before training combined with protein hydrolysates directly afterwards seem to be more reasonable [Strasser et al. 2018]. Protein hydrolysates are more readily used by the body than the proteins provided in a meal [Manninen 2006]. Notably, attention should be given to a balanced diet which supplies additional nutrients such as vitamin D, antioxidants, omega-3 fatty acids, and balances the acid-forming properties of protein with the appropriate intake of vegetables and fruits. A mixed diet such as the so-called Mediterranean diet is said to meet these requirements [Robinson et al 2017].
Properties of Protein Hydrolysates
The effect of protein on muscle building has been mainly studied for whey protein hydrolysates, which are rich in branched chain amino acids such as leucine. Leucine and its metabolites α-ketoisocaproate (α-KIC) and β-hydroxy-β-methylbutyrate (HMB) stimulate protein synthesis in muscles, inhibit protein degradation, and play a role in energy balance [Duan et al. 2016]. Collagen peptides with a lower content of branched chain amino acids have so far only been studied to a limited extent. One study showed that both muscle mass and muscle strength could be increased by administering Bioactive Collagen Peptides in conjunction with strength training, versus a placebo [Zdzieblik et al. 2015]. This might be due to collagen peptides showing a better nitrogen balance than whey protein [Hays et al. 2009]. In addition, collagen peptides are rich in glycine and arginine, which are needed for the generation of creatine phosphate, an important source of energy for muscle work [Berdanier 2015]. Although glycine is largely involved in human physiology, it is not an essential amino acid. However, it can become essential under certain circumstances [Razak et al. 2017], e.g., obesity and diabetes are associated with a disrupted glycine metabolism [Adeva-Andany et al. 2018]. Glycine is extremely important in stabilizing the collagen superhelix, and a deficiency can affect the functionality of blood vessels and other collagen structures such as connective tissue [Adeva-Andany 2018, Razak et al. 2017]. This may be due to the close interaction of muscle tissue with its surrounding connective tissue. The use of Bioactive Collagen Peptides also reduces joint pain caused by intensive training [Zdzieblik et al. 2017]. One could hypothesize that training results are improved by collagen peptides. However, this still has to be proven.
Clinical Studies
In a 12-week, double-blind randomized study, Zdzieblik and coworkers investigated the effect of Bioactive Collagen Peptides in combination with strength training on fat-free muscle mass and muscle strength in older volunteers suffering from age-related muscle atrophy (sarcopenia) [Zdzieblik et al. 2015]. The study included healthy men over 65 years of age with sarcopenia, a common Western diet, and adequate protein intake.
Over a period of 12 weeks, 53 volunteers underwent vigorous strength training three times a week for 60 minutes. Of those, 26 received 15 g Bioactive Collagen Peptides daily as powder dissolved in 250 ml water, whereas 27 controls received 15 g placebo (silicon dioxide). The participants were advised to take the powder within one hour after training, and to stick to that same time on resting days, if possible. After three months, fat-free muscle mass, muscle strength, and bone mass had increased, and fat mass decreased in all groups, whereas body weight was left almost unchanged, which suggests a pronounced training effect. The verum group, however, showed significantly higher effects than the placebo group, which indicates a positive influence of collagen peptides.
A review of non-drug interventions comes to the conclusion that strength and balance training, in particular, are beneficial to prevent age-related muscle loss (sarcopenia). There might also be a positive effect of additionally administered dietary supplements. No negative effects are expected to be induced by these interventions. Notably, further and carefully conducted studies are warranted [Lozano-Montoya et al. 2017].
A systematic review investigated if dietary supplementation with protein (amino acids, proteins, whey protein, collagen hydrolysate) still enhances the positive effects of strength and/or endurance training in individuals over 65 years suffering from sarcopenia [Luo et al. 2017]. The authors included studies from different countries and fields (outpatient and inpatient) to present a comprehensive view with participants of different origins and living conditions. The review concluded that dietary supplementation can improve muscle mass and strength training effects in older people with sarcopenia. Further studies are needed to clarify the characteristics and dosages of best performing products with respect to different patients, since many factors such as the dosage and the nutritional status may largely influence the results.
The extent to which supplementation with protein supplements can influence the negative impact of calorie restriction and reduced activity in older people has been studied in a randomized double-blind study which included 31 men and women aged 65 to 80 years [Oikawa et al. 2018]. During this study, 16 volunteers took whey protein and 15 volunteers took Bioactive Collagen Peptides at a dose of 30 g twice daily. In total, participants were observed for 5 weeks. In the first week, subjects received a diet with 0.8 g protein per kg of body weight without any supplementation. The diet of the subsequent week was restricted in calories, followed by two weeks with caloric reduction combined with reduced physical activity, supplemented with 1.6 g protein per kg of body weight. This intervention was followed by a one-week recovery period with adequate energy intake and with supplementation, completing the 5-week observational period. Despite the increased protein intake, both groups experienced a decrease in fat-free body mass during the energy restriction period as well as during the period with energy restriction and reduced physical activity. Loss of fat-free body mass seemed to be less pronounced in the collagen peptide group than in the whey protein group. Unfortunately, the authors of the study do not address these differences. The rate of muscle protein synthesis also decreased in both groups, and even though the increased synthesis of muscle protein during recovery phase was more pronounced in the whey protein group, it did not reach baseline. A prolonged observation period during the recovery period would have been reasonable. In addition, a possible impact on muscle function, which is an important parameter for clinical relevance, was not determined. All participants were heavily overweight, which is another limitation of the study. The high dose of collagen peptides, which corresponds to a multiple of the recommended dose, is also questionable, as sufficient supply of essential amino acids may not be achieved for some individuals. Unfortunately, clear information on this issue is missing as well.
Summary
Maintaining a good performance is important for quality of life in advancing age and may be influenced by multiple factors [Tieland et al. 2018]. Not only muscle mass, but also muscle functionality, such as strength, endurance, and joint and skeletal stability play an important role. It has clearly been shown, that physical activity and a sufficient supply of nutrients and energy are crucial factors. Since the anabolic metabolism of amino acids decreases in advancing age, an increased protein intake is recommended. However, even an increased protein intake cannot prevent the loss of muscle mass when calorie intake is reduced. Individuals with normal weight and well-trained individuals are more responsive to protein supplementation [Stokes et al. 2018]. Further studies are, therefore, needed to analyze optimal dosage for special situations in life, time of administration, and type of protein supplements.
Illustrative Material
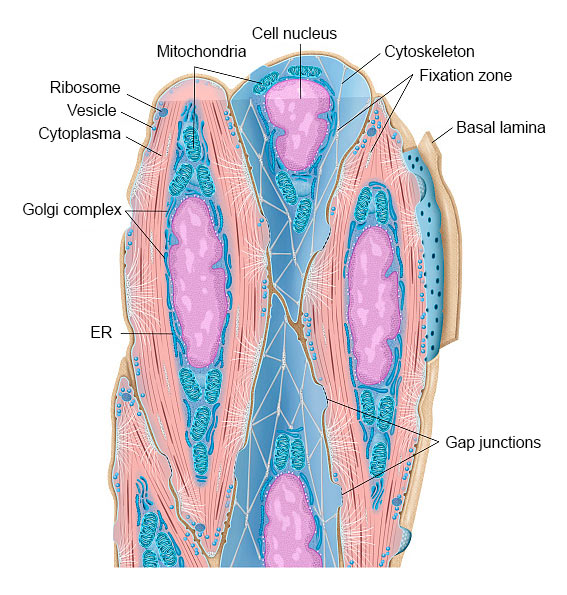
Muscle Tissue and Histology of Smooth Muscle Cells
Smooth muscles contain contractile tissue found mainly in the walls of all hollow organs, e.g., the intestine, respiratory tract, blood vessels, urinary tract, and reproductive organs.
The tissue is characterized by elongated, thin muscle cells (myocytes) without striation. The smooth spindle-shaped muscle cells contain elongated cell nuclei, glycogen stores, and cytoplastic organelles such as mitochondria, ER, ribosomes and Golgi complex.
Most of the cell’s cytoplasm is filled with actin microfilaments and myosin filaments. Each smooth muscle cell is surrounded by a basal lamina and a network of small bundles of collagen fibrils.
Source of figure: Frank Geisler (MediDesign)
Last update: December 2022